https://doi.org/10.1016/j.wasman.2022.10.003
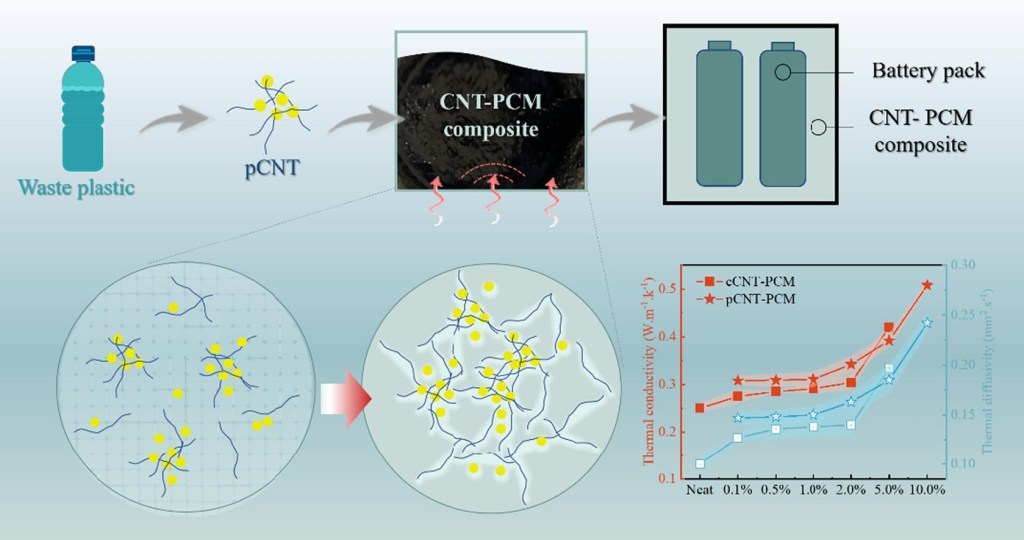
“Carbon nanotube (CNT), has been demonstrated as a promising high-value product from thermal chemical conversion of waste plastics and securing new applications is an important prerequisite for large-scale production of CNT from waste-plastic recycling. In this study, CNT, produced from waste plastic through chemical vapor deposition (pCNT), was applied as a nanofiller in phase change material (PCM), affording pCNT-PCM composites. Compared with pure PCM, the addition of 5.0 wt% pCNT rendered the peak melting temperature increase by 1.3 ℃, latent heat retain by 90.7%, and thermal conductivity increase by 104%. The results of morphological analysis and leakage testing confirmed that pCNT has similar PCM encapsulation performance and shape stability to those of commercial CNT. The formation of uniform pCNT cluster networks allowed for a large CNT loading into the PCM on the premise of free phase change, responsible for the high thermal conductivity inside the homogeneous phase. Thus, the resulting capillary forces retained a high latent heat capacity and suitable melting temperature and prohibited PCM leakage from the matrix to the outside during re-melting as the pCNT loading ratio increased. Therefore, the as-prepared pCNT-PCM composite is believed to have similar potential to cCNT and shows prominent performance as a flowable conductive filler for battery thermal management systems.”
“
Fig. 2 presents the TGA curves of pure PCM42, pure cCNT, pure pCNT, and two mass fractions for both cCNT-PCM and pCNT-PCM composites. In Fig. 2a, the first instance of mass loss, around 300 ℃, was due to paraffin degradation. Then, both cCNT and pCNT started to lose weight around 500 ℃ and stopped at 700 ℃. The synthesized pCNT was not purified, thereby partial Fe-based catalyst remained and was heat-stable at 800 ℃. Ultimately, the weight ratio of pCNT decreased and stablized at 40 %, approximately 35 % more than that of cCNT, thus the synthesized pCNT shows better thermal stability than cCNT. For the cCNT-PCM and pCNT-PCM composites, their weight loss is slower than pure PCM because CNT is known for resistance to thermal degradation. Notably, the curves of cCNT-PCM and pCNT-PCM composites nearly overlap at both 0.1 wt% and 5.0 wt% CNT loadings. The curves reveal similar thermal behaviour since the CNT dispersed well in a chemically inert PCM. Compared with cCNT, pCNT can impart greater thermal performance to PCM at a similar CNT loading. Meanwhile, with the increase of CNT loading, the weight loss of CNT-PCM is smaller, as shown in Fig. 2a, and the peak thermal decomposition temperature of PCM nanocomposites in Fig. 2b gradually moves to a slightly higher temperature. This result demonstrates that CNT promotes the global thermal stability of the PCM nanocomposite.

“


Leave a comment